
![]()
Department
of Chemistry
Health,
Safety and Environment
![]()
 Use Of Fire Extinguishers
Use Of Fire Extinguishers
Contents
- Definition of fire
- The Triangle of Fire
- Theory of fire extinguishing
- Classification of fires and appropriate methods for extinguishing
- Fire extinguishers
Fire is the whole of occurrences that accompany uncontrolled hazardous oxidation. It results from a chemical reaction between a fuel and oxygen and it is usually accompanied by heat and smoke generation and by emission of light or flames.
A fire can start only when the following elements are combined:
- a fuel, being a gas, a liquid or a solid,
- an oxidant, usually air oxygen (21% of air),
- a heat source, such as a flame, a spark or the like.
Together they form the so-called Triangle of Fire, where each leg represents a parameter.

Removal or elimination of one of the parameters prevents or ceases fire. Insight in these parameters allows for fire prevention and for efficient extinguishing of fire.
To understand fire prevention; it is important to recognize that every fire consists of a chemical reaction between a fuel and oxygen, activated by some form of energy.
With reference to the fire triangle, the principles of fire extinguishing and of different means for fire extinguishing can be deducted. Elimination of one of the three parameters 'fuel, oxygen, heat source) will halt the reaction. Every means of fire extinguishing destroys the triangle of fire.
Extinguishing a fire can be accomplished by:
- 1. Elimination
of the fuel
Lack of fuel will extinguish any fire. However, it is often almost impossible to remove fuel from an ongoing fire.
Examples where this means can be used effectively:
- Cutting the supply by closing a gas- or oil valve,
- Pumping liquid fuel from the bottom of the fuel tank to another reservoir,
- Spreading out the fuel: timber fires can be extinguished easier when the wood is spread out.
Most fires extinguish spontaneously when the oxygen level drops below 14%.
Preventing air or oxygen from reaching the fire will extinguish it. One way this can be accomplished is through partial replacement of the air by an inert gas, such as carbon dioxide (CO2), steam or nitrogen.This method is effective only for smaller liquid fuel fires using portable fire extinguishers. Class A fires cannot be treated this way.
Another way of using this "suffocation method" is by cutting the supply of oxygen to the fire. Covering a burning frying pan with a lid or with a moist towel will effectively stop the fire.
Examples of preventing oxygen from feeding the fire:
- Putting a lid on a burning recipient,
- Covering a fire with foam or powder , thus preventing generation of flammable fumes.
3. Elimination of the heat source - cooling downThis method is used most often. Cooling the fuel below its ignition temperature will cease the fire. Water is used most frequently to cool the fuel, as it boils at 100°C with a large heat of evaporation. This is the most efficient method for Class A fires.
Spraying sufficient amounts of water over a fire is more efficient than using a steady water jet.4. Neutralizing the chain reaction
Every fire involves a chain reaction, in which a multitude of chemical reactions generate chemical compounds or elements, that sustain the process. These elements are called "free radicals".
Some means of extinguishing, such as powders or halon, can scavenge these very reactive radicals, thus preventing propagation of the fire. These means have a negative catalytic effect on the fire.
Classification of fires and appropriate methods for extinguishing
The type of the fire and the properties of the burning materials determine the method of choice to extinguish the fire.
Fires are classified in different categories, that feature each appropriate methods of extinguishing.
Class A comprises fires of solid fuels, such as wood, paper, cardboard, coal, fabrics, etc. (burning dry matter).
Appropriate means of extinguishing:: water, foam or some special powders.
Class B comprises fires of liquid fuels - more precisely of the vapors in equilibrium with the liquids -, such as oil, gasoline, alcohol, paints, varnishes, tar, grease,Ö Contrary to Class A fires, Class B fires proceed at the surface of the fuel, where to a more or lesser degree the vapor phase of the liquid acts as the actual fuel.
Appropriate means of extinguishing:: powders, CO2, foam.
Class C comprises fires of flammable gases, such as natural gas, methane, propane, butane,Ö
Appropriate means of extinguishing:: FIRST OF ALL, THE GAS SUPPLY SHOULD BE CUT. The fire will extinguish automatically. If this is impossible, the fires can be kept under control by powders, CO2, halon. It is dangerous to extinguish the fire as long as the gas supply is not cut, for accumulation of gas may pose an explosion hazard.
Class D comprises fires of chemicals, such as flammable metals (magnesium, sodium, titanium,Ö).
Appropriate means of extinguishing: commonly used methods are inappropriate and may worsen things. Powders and dry sand are excellent materials.
Class E comprises fires in electrical apparatuses, such as motors, transformers, switch boards, cables,Ö
Appropriate means of extinguishing: NEVER USE CONDUCTIVE MATERIALS? SUCH AS WATER OR FOAMS. Preferred methods use powders, CO2 or halon. Occasionally non conductive high pressure methods can be used.
Knowledge of the procedures to operate fire extinguishers is important to enable swift reaction to a developing fire, before it becomes to big to handle.
Apparatuses containing powder or CO2 are idealto deal with beginning fires. These apparatuses are abundant in the laboratories. Please note they are effective only when used to extinguish small scale fires or developing fires.
Performance
Powder is forced out under pressure and forms a dense cloud around the burning mass. The powder contains approximately 80% of NaHCO3. When in contact with fire, it dissociates in CO2 and H2O, thus depleting the surrounding atmosphere from the oxygen necessary to sustain the fire and at the same time lowering the temperature by absorbing energy to dissociate. In addition, a layer of powder will be deposited on the mass. Direct contact with the surrounding air becomes impossible, thus preventing re-ignition of the fuel.
Powder fire extinguishers have a range of 6 to 8 meters, but the nozzle should not be more than 3 to 4 meters away from the fire to be effective.
![]() Procedure
Procedure
- Move the apparatus to the fire and pull or push down the safety pin to pressurize the extinguisher.
- Approach the fire as close as possible (dependent on the type and intensity of the fire) under the lee Ascertain an open escape route is available in case of need.
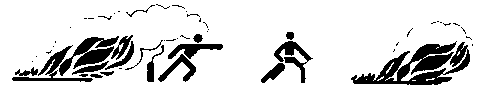
Wrong Right
- Point the funnel at the base of the flames and activate the lever. Use an uninterrupted jet of powder for Class B liquid fires and intermittent bursts of powder for Class A solid fires.
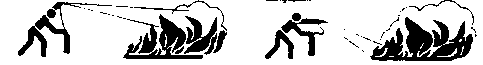
Wrong Right
- Under the protection of the powder cloud in front approach the fire to enhance the effectiveness of the method.
CO2-apparatuses don't leave traces as do
powder types. Hence they are more suitable to deal with
fires in rooms containing sensitive measuring and control
equipment, communication devices and the like. Powder spread
over the room requires thorough and expensive cleaning.
CO2 is an inert gas and disappears in the
atmosphere.
CO2 extinguishers have a limited effective range
of approximately 1.5 meters, forcing the operator to
approach the fire closely. In the case of electrical fires
where heavy smoke prevents the operator from clearly
distinguishing where exactly the fire source is located the
risk of electrocution by inadvertent contact of the metal
funnel with live leads turns this method inappropriate.
Performance
CO2 extinguishers contain CO2
gas under a pressure of approximately 60
kg/cm2. When released to atmospheric pressure,
the temperature of the gas drops to ñ79°C.
This temperature drop has a twofold effect. CO2
extinguishers are powerful weapons. Never engage in
horseplay or point the funnels to people or people's
faces. CO2 snow can freeze human tissue
and cause wounds comparable to third degree burns
On the one hand, the temperature of the burning mass can be lowered beneath the ignition temperature of the fuel, thus extinguishing the fire. More importantly however,
CO2 gas is inert and heavier than air, so it displaces the source of oxygen to the fire and builds a protective blanket over the fuel
![]() Procedure
Procedure
- Move the apparatus to the fire and remove the safety pin from the handle.
- Approach the fire as close as possible (dependent on the type and intensity of the fire) under the lee Ascertain an open escape route is available in case of need.
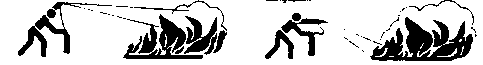
Wrong Right
- Point the funnel at the base of the flames from a distance of approximately 1.5 meters and activate the lever. Do not fire the gas jet from too close a distance at especially Class B liquid fires, as this may result in fuel splattering and fire propagation to adjacent areas. Always apply an uninterrupted jet of gas.
Water acts as a coolant. It has the most pronounced cooling effect of all extinguishing means through its high specific heat and low boiling point of 100°C. It succeeds in dropping the fuel temperature to below the ignition temperature, thus preventing further propagation of fire. Evaporation of water requires lots of energy to be delivered by the burning mass. Spraying water is more efficient than jetting, because a larger amount of water can come into contact with the fire and extract heat from it. Steam formation has a secondary effect of displacing oxygen, thus suffocating the fire. One liter of water can produce 1,650 liters of steam.
Advantages of water
- Lowest cost
- Abundance
- Not toxic
- Allows extinguishing from great distances
Disadvantages of water
- Can cause severe damage to properties
- Freezing point of 0°C
- Many fuels (paper, foamed plastics, fabrics) absorb water readily thereby increasing their weight
- Ineffective to even dangerous with flammable liquids, some metals, electrical fires
When a fire develops, time is of the essence.
Automated systems are most effective the sooner they are
activated upon onset of a fire..
- Systems based on powder
At K.U.Leuven this type is in use primarily in fireplaces and in engine rooms of elevators. Detection happens through fuses programmed at 70°c, 90°C or 120°C. When the fuses melt, powder is pressurized and spread over the surfaces to be protected. Simultaneously all power supply is cut.
Systems based on CO2
These systems are suited for rooms with high degree of occupation, such as electrical cabinets, engine rooms, computer rooms, Ö As they displace oxygen from the rooms, they must be inactivated prior to entering the room.
Systems based on halon
Due to their environmental hazards these systems are gradually replaced at K.U.Leuven.
As for CO2 systems the working principle is based on suffocation. Halon is a liquefied gas, freon 1301. A concentration of less than 5% is adequate as fire extinguisher, enabling personnel to enter the room without incurring health risks.
![]()

Copyright Katholieke Universiteit
Leuven
Information provider: Katholieke
Universiteit Leuven
Comments for the authors: Chris
Vinckier
Page design: Georges
Stevens
URL:
http://www.chem.kuleuven.ac.be/safety/info1.htm
Status: public -- Last revision: February
21, 2001